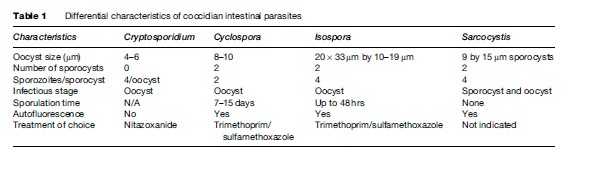
Diarrhea is a very common illness, especially in the developing world, and is frequently experienced by travelers. Cryptosporidium, Cyclospora, Isospora, Giardia, amoeba, and Sarcocystis are pathogenic protozoan parasites that can cause these gastrointestinal illnesses. Commensal parasites are also relatively common in developing countries and less frequently identified in the developed world. Worldwide, Giardia is the most common protozoan infection in the gastrointestinal tract of humans. It was probably first seen by Anton van Leuwenhoek in the late seventeenth century. In Tennessee, Giardia cysts have been identified in human feces from about 600 BC. Cryptosporidium and Giardia have also been reported from samples 500 to 3000 years old from the Andean regions in Peru, and from 4300to 1100-year-old samples from the coastal regions of Peru. Cryptosporidium became much more relevant to public health in the early 1980s with the emergence of the AIDS epidemic. Opportunistic and emerging parasitic infections also include Isospora, particularly in HIV and AIDS patients. Cyclospora has been observed in certain regions of the developing world; however, globalization of the food supply and increase in international travel have revealed that parasitic infections can also cause epidemics in the developed world.
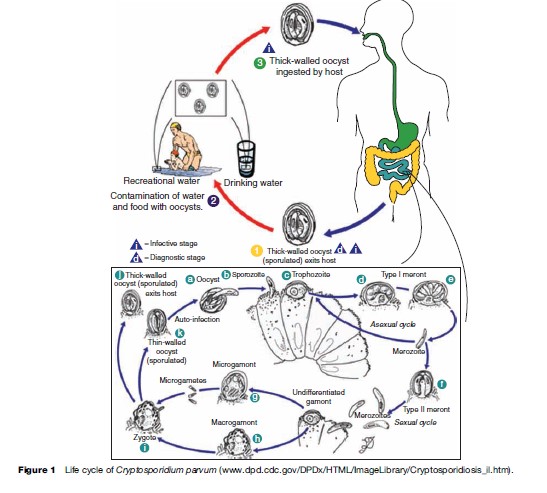
 Oocysts are excreted in the feces of an infected individual or, in the case of immunocompromised persons, the sputum. These oocysts are already infectious and can infect susceptible individuals. When ingested or inhaled, the oocysts undergo a process of excystation, in which four sporozoites per oocyst are released. These sporozoites invade the epithelial cells of the gastrointestinal (preferentially ileum) or respiratory epithelial cells. Within the host cell, Cryptosporidium undergoes a series of asexual multiplication producing meronts: type I (8 merozoites) and type II (4 merozoites). Asexual multiplication can continue, or organisms can differentiate to the sexual forms of the parasite. The zygote is formed after the microgametocyte fertilizes the macrogametocyte. The zygote will mature and differentiate to either thin-walled oocysts (20%), which can continue the reinfection in the intestine, or thick-walled oocysts (80%), which are environmentally resistant, and therefore can survive in water or foods (Figure 1).
Oocysts are excreted in the feces of an infected individual or, in the case of immunocompromised persons, the sputum. These oocysts are already infectious and can infect susceptible individuals. When ingested or inhaled, the oocysts undergo a process of excystation, in which four sporozoites per oocyst are released. These sporozoites invade the epithelial cells of the gastrointestinal (preferentially ileum) or respiratory epithelial cells. Within the host cell, Cryptosporidium undergoes a series of asexual multiplication producing meronts: type I (8 merozoites) and type II (4 merozoites). Asexual multiplication can continue, or organisms can differentiate to the sexual forms of the parasite. The zygote is formed after the microgametocyte fertilizes the macrogametocyte. The zygote will mature and differentiate to either thin-walled oocysts (20%), which can continue the reinfection in the intestine, or thick-walled oocysts (80%), which are environmentally resistant, and therefore can survive in water or foods (Figure 1).
 The localization of the Cryptosporidium parasitic vacuole is intracellular but extracytoplasmic. A feeding organelle is located between the parasite and the cytoplasmic membrane that allows selective transport of substances. This unique localization protects the parasite from the host’s cellular defense mechanisms and enables resistance to drug therapy. Cryptosporidium has three salvage enzymes for pyrimidines, which make it entirely dependent on the host. Two of these, uridine kinase-uracil phosphoribosyltransferase and thymidine kinase, are unique to C. parvum within the phylum Apicomplexa, and may account in part for the organism’s resistance to a broad range of drugs.
Many drugs have been evaluated against Cryptosporidium but only a handful have had some effectiveness. In the United States, the only FDA-approved drug for treatment of cryptosporidiosis is nitazoxanide (approved for use in children). When nitazoxanide (500 mg) is given twice daily for 3 days to children without HIV infection, a 96% clinical recovery is achieved, and 93% of patients stop shedding Cryptosporidium oocysts. Paromomycin has also been evaluated with conflicting results, particularly in AIDS patients. In HIV-infected persons, highly active antiretroviral treatment (HAART) is the most effective means of resolving Cryptosporidium infection as it aids in the replenishment of CD4þ cells. Relapse of the infection may occur if treatment is discontinued.
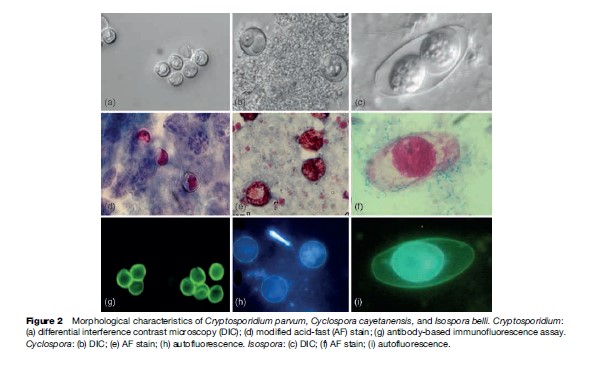
C. parvum oocysts measure 4 to 6 mm in diameter and can be identified by examining fecal or sputum samples using the modified acid-fast stain (Figure 2). Other antibody-based assays – including direct and indirect immunofluorescence, and enzyme immunoassays – are commercially available for use with clinical and environmental samples. Molecular assays are also used for diagnosis, genetic fingerprinting, and genotyping.
The localization of the Cryptosporidium parasitic vacuole is intracellular but extracytoplasmic. A feeding organelle is located between the parasite and the cytoplasmic membrane that allows selective transport of substances. This unique localization protects the parasite from the host’s cellular defense mechanisms and enables resistance to drug therapy. Cryptosporidium has three salvage enzymes for pyrimidines, which make it entirely dependent on the host. Two of these, uridine kinase-uracil phosphoribosyltransferase and thymidine kinase, are unique to C. parvum within the phylum Apicomplexa, and may account in part for the organism’s resistance to a broad range of drugs.
Many drugs have been evaluated against Cryptosporidium but only a handful have had some effectiveness. In the United States, the only FDA-approved drug for treatment of cryptosporidiosis is nitazoxanide (approved for use in children). When nitazoxanide (500 mg) is given twice daily for 3 days to children without HIV infection, a 96% clinical recovery is achieved, and 93% of patients stop shedding Cryptosporidium oocysts. Paromomycin has also been evaluated with conflicting results, particularly in AIDS patients. In HIV-infected persons, highly active antiretroviral treatment (HAART) is the most effective means of resolving Cryptosporidium infection as it aids in the replenishment of CD4þ cells. Relapse of the infection may occur if treatment is discontinued.
C. parvum oocysts measure 4 to 6 mm in diameter and can be identified by examining fecal or sputum samples using the modified acid-fast stain (Figure 2). Other antibody-based assays – including direct and indirect immunofluorescence, and enzyme immunoassays – are commercially available for use with clinical and environmental samples. Molecular assays are also used for diagnosis, genetic fingerprinting, and genotyping.
 Although Cryptosporidium has been previously identified worldwide, the availability of molecular tools in recent years has enabled the determination of the prevalence of different species and genotypes in various parts of the world. Cryptosporidium has been isolated from various species of animals other than their natural host. Flies, rotifers, and free-living nematodes may serve as transport vectors of viable oocysts. Mussels, clams, and oysters can concentrate Cryptosporidium oocysts in their gills and hemolymph and have been isolated from shellfish worldwide. However, to date, outbreaks of cryptosporidiosis associated with consumption of shellfish have not been reported.
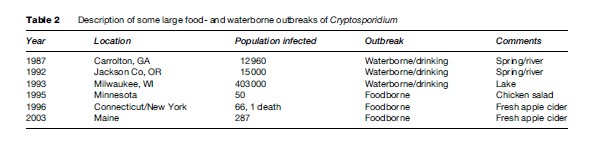
In parts of the developing world, where access to clean water and adequate sanitation are lacking, cryptosporidiosis is endemic, and young children are most severely affected. In the United States, investigations of foodborne outbreaks have implicated consumption of fresh vegetables and prepared foods (Table 2). Vegetables and produce eaten raw may be contaminated by irrigation water (containing Cryptosporidium oocysts) at the farm level, by water used to keep produce fresh and hydrated at the market level, or during food preparation at the consumer level. In other instances, manipulation of foods by food handlers with cryptosporidiosis or improper hygienic practices has initiated a foodborne outbreak.
Although Cryptosporidium has been previously identified worldwide, the availability of molecular tools in recent years has enabled the determination of the prevalence of different species and genotypes in various parts of the world. Cryptosporidium has been isolated from various species of animals other than their natural host. Flies, rotifers, and free-living nematodes may serve as transport vectors of viable oocysts. Mussels, clams, and oysters can concentrate Cryptosporidium oocysts in their gills and hemolymph and have been isolated from shellfish worldwide. However, to date, outbreaks of cryptosporidiosis associated with consumption of shellfish have not been reported.
In parts of the developing world, where access to clean water and adequate sanitation are lacking, cryptosporidiosis is endemic, and young children are most severely affected. In the United States, investigations of foodborne outbreaks have implicated consumption of fresh vegetables and prepared foods (Table 2). Vegetables and produce eaten raw may be contaminated by irrigation water (containing Cryptosporidium oocysts) at the farm level, by water used to keep produce fresh and hydrated at the market level, or during food preparation at the consumer level. In other instances, manipulation of foods by food handlers with cryptosporidiosis or improper hygienic practices has initiated a foodborne outbreak.
 Waterborne transmission, either by drinking or recreational water, has proven to be the most frequent mode of transmission of this parasite. Cryptosporidium oocysts are highly resistant to chlorine at levels normally used to treat drinking water or chlorinated recreational water venues. In the United States, many Cryptosporidium waterborne outbreaks have been reported (see Table 2), and in recent years, swimming pools and other recreational water sites have been the source of infection. Outbreaks have occurred in swimming pools that were not properly maintained or treated regularly to inactivate potential contaminants. Much emphasis has been placed on preventing parasite transmission by way of tap or drinking water. The water industry has studied extensively procedures to adequately remove and inactivate Cryptosporidium oocysts. Regulations and methods to detect this parasite in water are available from the U.S. Environmental Protection Agency (see the section ‘Relevant Websites’).
Transmission may also occur via person-to-person contact (within households, among children in day care centers, between sexual partners) or by contact with animals excreting Cryptosporidium oocysts. Petting zoos have been implicated in Cryptosporidium outbreaks involving children who touched farm animals and did not properly clean their hands afterward.
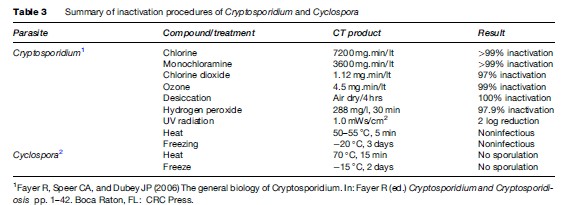
Cryptosporidiosis can be prevented by maintaining good hygienic practices, including washing hands after using toilets and before handling food. Special attention is needed for children and persons who contact animals. This is particularly important for food handlers. Hikers should avoid drinking river or lake water unless it is boiled, filtered (< 2 mm pore diameter), or, though more difficult, rendered safe by efficient chemical treatment. Water coagulation/flocculation, sedimentation, filtration, and disinfection can remove more than 99% of oocysts. UV, ozone, chlorine, and chlorine dioxide have been examined for inactivation efficiency in Cryptosporidium with variable results (Table 3).
Waterborne transmission, either by drinking or recreational water, has proven to be the most frequent mode of transmission of this parasite. Cryptosporidium oocysts are highly resistant to chlorine at levels normally used to treat drinking water or chlorinated recreational water venues. In the United States, many Cryptosporidium waterborne outbreaks have been reported (see Table 2), and in recent years, swimming pools and other recreational water sites have been the source of infection. Outbreaks have occurred in swimming pools that were not properly maintained or treated regularly to inactivate potential contaminants. Much emphasis has been placed on preventing parasite transmission by way of tap or drinking water. The water industry has studied extensively procedures to adequately remove and inactivate Cryptosporidium oocysts. Regulations and methods to detect this parasite in water are available from the U.S. Environmental Protection Agency (see the section ‘Relevant Websites’).
Transmission may also occur via person-to-person contact (within households, among children in day care centers, between sexual partners) or by contact with animals excreting Cryptosporidium oocysts. Petting zoos have been implicated in Cryptosporidium outbreaks involving children who touched farm animals and did not properly clean their hands afterward.
Cryptosporidiosis can be prevented by maintaining good hygienic practices, including washing hands after using toilets and before handling food. Special attention is needed for children and persons who contact animals. This is particularly important for food handlers. Hikers should avoid drinking river or lake water unless it is boiled, filtered (< 2 mm pore diameter), or, though more difficult, rendered safe by efficient chemical treatment. Water coagulation/flocculation, sedimentation, filtration, and disinfection can remove more than 99% of oocysts. UV, ozone, chlorine, and chlorine dioxide have been examined for inactivation efficiency in Cryptosporidium with variable results (Table 3).
 Trimethoprim/sulfamethoxazole (adults: 160–800 mg twice daily for 7 days; children: 5 mg/kg twice daily for 7 days) is effective for treatment of Cyclospora infection. Oocyst excretion and symptoms usually resolve 1 to 3 days post treatment. Ciprofloxacin has also been reported as an alternative treatment in sulfa-sensitive patients. Recurrence of infection in HIV-infected patients is more common and may require prolonged therapy.
Trimethoprim/sulfamethoxazole (adults: 160–800 mg twice daily for 7 days; children: 5 mg/kg twice daily for 7 days) is effective for treatment of Cyclospora infection. Oocyst excretion and symptoms usually resolve 1 to 3 days post treatment. Ciprofloxacin has also been reported as an alternative treatment in sulfa-sensitive patients. Recurrence of infection in HIV-infected patients is more common and may require prolonged therapy.
Intestinal Coccidia
Cryptosporidiosis
Gastrointestinal cryptosporidiosis is characterized by profuse watery diarrhea, which is more severe in children, the elderly, and individuals with compromised immunity. Although Cryptosporidium has been identified primarily in the gastrointestinal tract as a cause of diarrheal illness in humans, AIDS patients have also been reported with infections of the gallbladder and the respiratory tract. In immunocompetent individuals, diarrhea lasts, on average, 15 days; however, oocyst shedding in the feces can continue for more than 30 days. In immunocompromised patients, diarrhea is profuse and can last for months. Interferon gamma seems to play a role in controlling the infection. The clinical presentation of cryptosporidiosis has been variable, and in the past, infection was considered to be caused by a unique species, Cryptosporidium parvum; this was later differentiated as genotype I (or human genotype) and II (or bovine genotype). Genotype I was later renamed C. hominis, and C. parvum (genotype II) is still considered the zoonotic group and consists of various genotypes. Of the 15 species of Cryptosporidium described as infecting reptiles, fish, birds, and mammals, eight can also infect humans: C. hominis (of humans), C. parvum (of cattle), C. canis (of dogs), C. felis (of cats), C. meleagridis (of turkeys), C. muris (of rodents), C. suis (of pigs), and Cryptosporidium cervine genotype. Most of these are morphologically similar (Table 1); therefore, molecular assays are needed to differentiate between them. Oocysts are excreted in the feces of an infected individual or, in the case of immunocompromised persons, the sputum. These oocysts are already infectious and can infect susceptible individuals. When ingested or inhaled, the oocysts undergo a process of excystation, in which four sporozoites per oocyst are released. These sporozoites invade the epithelial cells of the gastrointestinal (preferentially ileum) or respiratory epithelial cells. Within the host cell, Cryptosporidium undergoes a series of asexual multiplication producing meronts: type I (8 merozoites) and type II (4 merozoites). Asexual multiplication can continue, or organisms can differentiate to the sexual forms of the parasite. The zygote is formed after the microgametocyte fertilizes the macrogametocyte. The zygote will mature and differentiate to either thin-walled oocysts (20%), which can continue the reinfection in the intestine, or thick-walled oocysts (80%), which are environmentally resistant, and therefore can survive in water or foods (Figure 1).
Oocysts are excreted in the feces of an infected individual or, in the case of immunocompromised persons, the sputum. These oocysts are already infectious and can infect susceptible individuals. When ingested or inhaled, the oocysts undergo a process of excystation, in which four sporozoites per oocyst are released. These sporozoites invade the epithelial cells of the gastrointestinal (preferentially ileum) or respiratory epithelial cells. Within the host cell, Cryptosporidium undergoes a series of asexual multiplication producing meronts: type I (8 merozoites) and type II (4 merozoites). Asexual multiplication can continue, or organisms can differentiate to the sexual forms of the parasite. The zygote is formed after the microgametocyte fertilizes the macrogametocyte. The zygote will mature and differentiate to either thin-walled oocysts (20%), which can continue the reinfection in the intestine, or thick-walled oocysts (80%), which are environmentally resistant, and therefore can survive in water or foods (Figure 1).
 The localization of the Cryptosporidium parasitic vacuole is intracellular but extracytoplasmic. A feeding organelle is located between the parasite and the cytoplasmic membrane that allows selective transport of substances. This unique localization protects the parasite from the host’s cellular defense mechanisms and enables resistance to drug therapy. Cryptosporidium has three salvage enzymes for pyrimidines, which make it entirely dependent on the host. Two of these, uridine kinase-uracil phosphoribosyltransferase and thymidine kinase, are unique to C. parvum within the phylum Apicomplexa, and may account in part for the organism’s resistance to a broad range of drugs.
Many drugs have been evaluated against Cryptosporidium but only a handful have had some effectiveness. In the United States, the only FDA-approved drug for treatment of cryptosporidiosis is nitazoxanide (approved for use in children). When nitazoxanide (500 mg) is given twice daily for 3 days to children without HIV infection, a 96% clinical recovery is achieved, and 93% of patients stop shedding Cryptosporidium oocysts. Paromomycin has also been evaluated with conflicting results, particularly in AIDS patients. In HIV-infected persons, highly active antiretroviral treatment (HAART) is the most effective means of resolving Cryptosporidium infection as it aids in the replenishment of CD4þ cells. Relapse of the infection may occur if treatment is discontinued.
C. parvum oocysts measure 4 to 6 mm in diameter and can be identified by examining fecal or sputum samples using the modified acid-fast stain (Figure 2). Other antibody-based assays – including direct and indirect immunofluorescence, and enzyme immunoassays – are commercially available for use with clinical and environmental samples. Molecular assays are also used for diagnosis, genetic fingerprinting, and genotyping.
The localization of the Cryptosporidium parasitic vacuole is intracellular but extracytoplasmic. A feeding organelle is located between the parasite and the cytoplasmic membrane that allows selective transport of substances. This unique localization protects the parasite from the host’s cellular defense mechanisms and enables resistance to drug therapy. Cryptosporidium has three salvage enzymes for pyrimidines, which make it entirely dependent on the host. Two of these, uridine kinase-uracil phosphoribosyltransferase and thymidine kinase, are unique to C. parvum within the phylum Apicomplexa, and may account in part for the organism’s resistance to a broad range of drugs.
Many drugs have been evaluated against Cryptosporidium but only a handful have had some effectiveness. In the United States, the only FDA-approved drug for treatment of cryptosporidiosis is nitazoxanide (approved for use in children). When nitazoxanide (500 mg) is given twice daily for 3 days to children without HIV infection, a 96% clinical recovery is achieved, and 93% of patients stop shedding Cryptosporidium oocysts. Paromomycin has also been evaluated with conflicting results, particularly in AIDS patients. In HIV-infected persons, highly active antiretroviral treatment (HAART) is the most effective means of resolving Cryptosporidium infection as it aids in the replenishment of CD4þ cells. Relapse of the infection may occur if treatment is discontinued.
C. parvum oocysts measure 4 to 6 mm in diameter and can be identified by examining fecal or sputum samples using the modified acid-fast stain (Figure 2). Other antibody-based assays – including direct and indirect immunofluorescence, and enzyme immunoassays – are commercially available for use with clinical and environmental samples. Molecular assays are also used for diagnosis, genetic fingerprinting, and genotyping.
 Although Cryptosporidium has been previously identified worldwide, the availability of molecular tools in recent years has enabled the determination of the prevalence of different species and genotypes in various parts of the world. Cryptosporidium has been isolated from various species of animals other than their natural host. Flies, rotifers, and free-living nematodes may serve as transport vectors of viable oocysts. Mussels, clams, and oysters can concentrate Cryptosporidium oocysts in their gills and hemolymph and have been isolated from shellfish worldwide. However, to date, outbreaks of cryptosporidiosis associated with consumption of shellfish have not been reported.
In parts of the developing world, where access to clean water and adequate sanitation are lacking, cryptosporidiosis is endemic, and young children are most severely affected. In the United States, investigations of foodborne outbreaks have implicated consumption of fresh vegetables and prepared foods (Table 2). Vegetables and produce eaten raw may be contaminated by irrigation water (containing Cryptosporidium oocysts) at the farm level, by water used to keep produce fresh and hydrated at the market level, or during food preparation at the consumer level. In other instances, manipulation of foods by food handlers with cryptosporidiosis or improper hygienic practices has initiated a foodborne outbreak.
Although Cryptosporidium has been previously identified worldwide, the availability of molecular tools in recent years has enabled the determination of the prevalence of different species and genotypes in various parts of the world. Cryptosporidium has been isolated from various species of animals other than their natural host. Flies, rotifers, and free-living nematodes may serve as transport vectors of viable oocysts. Mussels, clams, and oysters can concentrate Cryptosporidium oocysts in their gills and hemolymph and have been isolated from shellfish worldwide. However, to date, outbreaks of cryptosporidiosis associated with consumption of shellfish have not been reported.
In parts of the developing world, where access to clean water and adequate sanitation are lacking, cryptosporidiosis is endemic, and young children are most severely affected. In the United States, investigations of foodborne outbreaks have implicated consumption of fresh vegetables and prepared foods (Table 2). Vegetables and produce eaten raw may be contaminated by irrigation water (containing Cryptosporidium oocysts) at the farm level, by water used to keep produce fresh and hydrated at the market level, or during food preparation at the consumer level. In other instances, manipulation of foods by food handlers with cryptosporidiosis or improper hygienic practices has initiated a foodborne outbreak.
 Waterborne transmission, either by drinking or recreational water, has proven to be the most frequent mode of transmission of this parasite. Cryptosporidium oocysts are highly resistant to chlorine at levels normally used to treat drinking water or chlorinated recreational water venues. In the United States, many Cryptosporidium waterborne outbreaks have been reported (see Table 2), and in recent years, swimming pools and other recreational water sites have been the source of infection. Outbreaks have occurred in swimming pools that were not properly maintained or treated regularly to inactivate potential contaminants. Much emphasis has been placed on preventing parasite transmission by way of tap or drinking water. The water industry has studied extensively procedures to adequately remove and inactivate Cryptosporidium oocysts. Regulations and methods to detect this parasite in water are available from the U.S. Environmental Protection Agency (see the section ‘Relevant Websites’).
Transmission may also occur via person-to-person contact (within households, among children in day care centers, between sexual partners) or by contact with animals excreting Cryptosporidium oocysts. Petting zoos have been implicated in Cryptosporidium outbreaks involving children who touched farm animals and did not properly clean their hands afterward.
Cryptosporidiosis can be prevented by maintaining good hygienic practices, including washing hands after using toilets and before handling food. Special attention is needed for children and persons who contact animals. This is particularly important for food handlers. Hikers should avoid drinking river or lake water unless it is boiled, filtered (< 2 mm pore diameter), or, though more difficult, rendered safe by efficient chemical treatment. Water coagulation/flocculation, sedimentation, filtration, and disinfection can remove more than 99% of oocysts. UV, ozone, chlorine, and chlorine dioxide have been examined for inactivation efficiency in Cryptosporidium with variable results (Table 3).
Waterborne transmission, either by drinking or recreational water, has proven to be the most frequent mode of transmission of this parasite. Cryptosporidium oocysts are highly resistant to chlorine at levels normally used to treat drinking water or chlorinated recreational water venues. In the United States, many Cryptosporidium waterborne outbreaks have been reported (see Table 2), and in recent years, swimming pools and other recreational water sites have been the source of infection. Outbreaks have occurred in swimming pools that were not properly maintained or treated regularly to inactivate potential contaminants. Much emphasis has been placed on preventing parasite transmission by way of tap or drinking water. The water industry has studied extensively procedures to adequately remove and inactivate Cryptosporidium oocysts. Regulations and methods to detect this parasite in water are available from the U.S. Environmental Protection Agency (see the section ‘Relevant Websites’).
Transmission may also occur via person-to-person contact (within households, among children in day care centers, between sexual partners) or by contact with animals excreting Cryptosporidium oocysts. Petting zoos have been implicated in Cryptosporidium outbreaks involving children who touched farm animals and did not properly clean their hands afterward.
Cryptosporidiosis can be prevented by maintaining good hygienic practices, including washing hands after using toilets and before handling food. Special attention is needed for children and persons who contact animals. This is particularly important for food handlers. Hikers should avoid drinking river or lake water unless it is boiled, filtered (< 2 mm pore diameter), or, though more difficult, rendered safe by efficient chemical treatment. Water coagulation/flocculation, sedimentation, filtration, and disinfection can remove more than 99% of oocysts. UV, ozone, chlorine, and chlorine dioxide have been examined for inactivation efficiency in Cryptosporidium with variable results (Table 3).

Cyclosporiasis
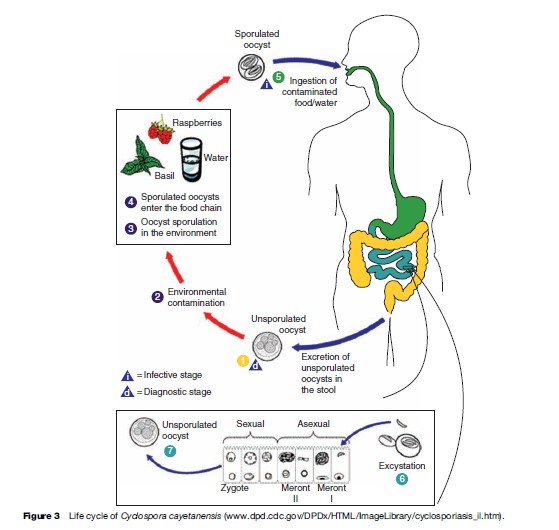
Cyclospora cayetanensis was first reported in patients with acute diarrhea in 1979. At that time it was considered to be a coccidian-like or a cyanobacteria-like organism. In 1992, it was finally fully characterized and classified within the phylum Apicomplexa and coccidian. Unlike other coccidian parasites that infect humans, Cyclospora requires 7 to 15 days to fully sporulate and become infectious. Cyclosporiasis is characterized by persistent diarrhea, anorexia, nausea, abdominal pain, flatulence, and in some instances, fever. As of today, Cyclospora cayetanensis is the only species described as infecting humans. Nonhuman primates also harbor other species of Cyclospora: C. cercopitheci, C. colobi, and C. papionis, which are morphologically similar to C. cayetanensis. These species seem to be also host-species specific. Molecular analysis of the 18S sRNA gene of C. cayetanensis and the three other Cyclospora species suggests that they are molecularly different, and that the genus Cyclospora is phylogenetically most closely related to the Eimeria species, although they are morphologically different. To date, none of the Cyclospora species have been propagated experimentally using animal models or human volunteers. Cyclospora oocysts measure 8–10 mm in diameter and stain variably using the modified acid-fast stain. Oocysts stain best when using a heated safranin stain protocol. Oocysts can be easily identified using phase contrast microscopy or by epifluorescence microscopy because the oocysts are autofluorescent. The last method is the most sensitive diagnostic assay in clinical specimens (see Figure 2). Unsporulated oocysts are excreted into the environment in the feces of infected individuals. After 7 to 15 days of incubation at 23 oC, the oocysts differentiate, and form two sporocysts per oocyst. Each of these sporocysts contains two sporozoites (see Figure 2). Cyclospora infection is acquired when contaminated food or water containing oocysts are ingested. Oocysts excyst and sporozoites are released and infect the epithelial cells of the intestinal tract, particularly the terminal portion of the jejunum and ileum. Sporozoites multiply asexually producing type I and II meronts. Asexual multiplication may continue or sexual reproduction may initiate and produce oocysts that are excreted in the feces of infected individuals (Figure 3). Trimethoprim/sulfamethoxazole (adults: 160–800 mg twice daily for 7 days; children: 5 mg/kg twice daily for 7 days) is effective for treatment of Cyclospora infection. Oocyst excretion and symptoms usually resolve 1 to 3 days post treatment. Ciprofloxacin has also been reported as an alternative treatment in sulfa-sensitive patients. Recurrence of infection in HIV-infected patients is more common and may require prolonged therapy.
Trimethoprim/sulfamethoxazole (adults: 160–800 mg twice daily for 7 days; children: 5 mg/kg twice daily for 7 days) is effective for treatment of Cyclospora infection. Oocyst excretion and symptoms usually resolve 1 to 3 days post treatment. Ciprofloxacin has also been reported as an alternative treatment in sulfa-sensitive patients. Recurrence of infection in HIV-infected patients is more common and may require prolonged therapy.
