Certain other serovars – for example, Blegdam, Bredeney, Cholerae-suis, Dublin, and Virchow – are also invasive but tend to cause pyemic infections and to localize in the viscera, meninges, bones, joints, and serous cavities. In developed countries many other serovars may also be invasive in certain circumstances, often related to the infective dose and the host. For instance, invasive disease by serovars generally regarded as noninvasive frequently occurs in immunocompromised patients or in patients with other underlying diseases. In developing countries a different picture has emerged, with serovars normally regarded as noninvasive – for example, Typhimurium, Wien, and Senftenberg being associated with highly virulent infections with a high degree of morbidity and mortality. Such infections have been associated with the carriage of plasmids that may enhance the invasive potential of their host organism (see the section ‘Developing countries’ under ‘Nontyphoidal salmonellas’).
Gastroenteritis
The most common symptoms following infection with the ubiquitous nontyphoidal serovars found in a number of animal species are those of an acute but mild to moderate enteritis with a short incubation period of 12–48 hours, occasionally as long as 4 days. As with the majority of gastrointestinal bacterial pathogens, symptoms can be more severe in vulnerable patient groups such as young children, the elderly, debilitated, and immunocompromised patients.Treatment
For enteric fever, including infections with S. Typhi and S. Paratyphi A and B, treatment with an appropriate antibiotic is essential and can be life-saving. As treatment may commence before the results of antimicrobial sensitivities are known it is important to be aware of options and possible problems before beginning treatment (see the section ‘Antimicrobial drug resistance’). The same strictures apply for invasive infections with nontyphoidal salmonellae. Multiple resistance, often including resistance to ‘critical’ antimicrobials, is becoming increasingly common for certain serovars and although not considered important for uncomplicated gastroenteritis, can be very important should extraintestinal spread occur. For uncomplicated gastroenteritis rehydration therapy is considered appropriate in most cases.Epidemiology
On a global scale it has been estimated that salmonella is responsible for an estimated 3 billion human infections each year. The World Health Organization (WHO) has estimated that typhoid fever accounts for 22 million of these cases and is responsible for 200 000 deaths annually (Crump et al., 2004).Typhoid And Paratyphoid Fever
Typhoid fever is a significant cause of morbidity and mortality among children and adults in developing countries. The organism remains endemic in developing countries in Africa, South and Central America, and the Indian subcontinent. The disease is also commonly reported from the Middle East, and some countries in Southern and Eastern Europe. In contrast, in developed countries such as the UK or the United States, the incidence of S. Typhi is much lower, with the majority of cases in travelers returning from endemic areas. For example, in the UK, between 150 and 300 cases occur each year with at least 70% of cases in patients with a history of recent foreign travel. Similarly, in the United States, 293 infections were reported in the 12-month period from 1 June 1996 to 31 May 1997, of which 81% were recorded in patients with a history of recent travel to endemic areas. The epidemiology of paratyphoid fever is less well documented than that of typhoid. Nevertheless, the WHO has estimated that up to 25% of enteric fevers may be caused by S. Paratyphi A and the disease is becoming increasingly common in several countries in South-East Asia, including Vietnam, India, and Nepal.Nontyphoidal Salmonellas
Data on infections caused by nontyphoidal salmonellas are difficult to quantify, mainly because of differences in surveillance systems or, indeed, the complete lack of such systems in many countries.Developed countries
Within the European Union (EU) studies by the Enternet surveillance network have provided data for about 150 000 human infections annually, with approximately 1000 deaths, from figures submitted by the 22 countries that report into Enter-net. In the United States, it has been estimated that there are approximately 200 000 infections annually. For the last two decades the most common serovar in cases of infection with nontyphoidal salmonellas in the EU has been S. Enteritidis (Saheed et al., 1999). This serovar has its reservoir in poultry, and from 1987 to 2000 the most common phage type (PT) within the serovar was PT 4. From 1987 to 2000 it was estimated that PT 4 was responsible for over 500 000 cases of S. Enteritidis in the United Kingdom alone (Figure 1). S. Enteritidis PT 4 was unusual in that the organism was transmitted vertically through the oviduct of infected birds, and many infections in humans were traced to infected eggs. Outbreaks were compounded by improper cooking techniques and by the storage at ambient temperatures of dishes made from raw eggs. Despite instructions on the cooking and handling of eggs, the outbreak was only contained by the vaccination of poultry flocks, namely breeding flocks in 1994, and commercial layers in 1998. Regrettably, since 2000, a considerable number of infections caused by non-PT 4 strains have been increasingly identified in the UK. A well-documented series of outbreak investigations and laboratory studies, supplemented by surveys of eggs on retail sale, have traced the source of these strains to eggs imported into the UK from Spain. As a result of various actions, including educating restauranteurs and also informing appropriate authorities within Spain, infections with these non-PT 4 strains have shown a marked decline since 2004.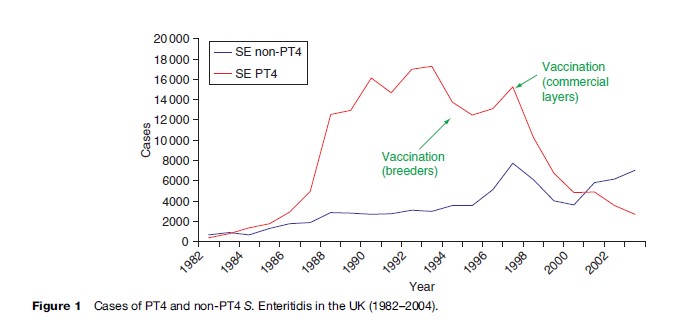 In the United States, similar problems with S. Enteritidis have been encountered, traced back to contaminated egg dishes. In contrast to the United Kingdom, the predominant PTs were 8 and 13a, although outbreaks of PT 4 not associated with foreign travel have been recognized since 1993.
Other serovars with an international distribution in developed countries have included S. Typhimurium definitive phage type (DT) 104. This organism exhibits chromosomally mediated multiple resistance (MR) to five antimicrobials – ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracyclines (R-type ACSSuT). MR S. Typhimurium DT 104 of R-type ACSSuT was first identified in the UK in the early 1980s from gulls and exotic birds. With the exception of a small outbreak in Scotland in the mid-1980s there were no isolations from humans until 1989, by which time MR S. Typhimurium DT 104 had also been isolated from cattle. Over the next 5 years this strain became epidemic in bovine animals throughout the UK, and also in poultry, particularly turkeys, and in pigs and sheep. Human infection with MR S. Typhimurium DT 104 has been associated with the consumption of chicken, beef, pork sausages, and meat paste and to a lesser extent with occupational contact with infected animals. This particular clone has subsequently caused outbreaks of infection in food animals and humans in numerous European countries and as far afield as South Africa, the United Arab Emirates, and the Philippines (Threlfall, 2000). In 1996 infections with MR DT 104 were recognized in cattle and humans in North America, both in Canada and in the United States. Of particular concern has been the resistance of the organism to a wide range of therapeutic antimicrobials. Furthermore, in some countries there have been reports of an apparent predilection of the organism to cause serious disease, although this is not the case in the UK.
In the United States, closely related strains of MR S. Newport with plasmid-encoded resistance to ceftriaxone have been associated with numerous infections in both cattle and humans. This has caused it to become the third most common serotype causing salmonellosis in man in the United States from 2000 to 2002. The organism commonly shows resistance to ACSSuT with additional resistance to third-generation cephalosporins mediated by the CMY-2 beta-lactamase gene (see ‘Developed countries’ under ‘Other salmonella serovars’).
Because of the association of many nontyphoidal salmonella serovars with food-production animals, many outbreaks have been linked to foods of animal origin or to foods contaminated with animal waste. This, coupled with the massive importation of foods between countries, including both developed and developing countries, has ensured that products contaminated with salmonella organisms have been widely distributed. An enormous diversity of vehicles of infection have been identified, ranging from salad vegetables to spices to coconut, as well as traditional animal-associated products such as poultry and poultry products, milk, cheese, and undercooked beef and pork. The EU-funded Enter-net surveillance network has identified over 50 such food-borne salmonella outbreaks since its inception in 1992 (Fisher and Threlfall, 2005; Cook et al., 2006), and, in many cases, has been instrumental in the development of intervention measures that have either contained existing outbreaks or have resulted in the removal of contaminated products from the food chain.
A further significant source of salmonellosis in developed countries is pet reptiles. Many reptiles carry salmonella organisms as part of their normal bacterial flora and, although the types of salmonella in reptiles are not those normally associated with large outbreaks, such as those caused by S. Enteritidis, the reptile-associated types can cause very serious illness, particularly in vulnerable patient groups such as young children or the elderly. There have been many cases of reptile-associated salmonellosis documented worldwide. In the United States in the 1970s, a large outbreak involving several thousand people was attributed to turtles; as a result, turtle hatching was banned in the United States in 1975.
In the United States, similar problems with S. Enteritidis have been encountered, traced back to contaminated egg dishes. In contrast to the United Kingdom, the predominant PTs were 8 and 13a, although outbreaks of PT 4 not associated with foreign travel have been recognized since 1993.
Other serovars with an international distribution in developed countries have included S. Typhimurium definitive phage type (DT) 104. This organism exhibits chromosomally mediated multiple resistance (MR) to five antimicrobials – ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracyclines (R-type ACSSuT). MR S. Typhimurium DT 104 of R-type ACSSuT was first identified in the UK in the early 1980s from gulls and exotic birds. With the exception of a small outbreak in Scotland in the mid-1980s there were no isolations from humans until 1989, by which time MR S. Typhimurium DT 104 had also been isolated from cattle. Over the next 5 years this strain became epidemic in bovine animals throughout the UK, and also in poultry, particularly turkeys, and in pigs and sheep. Human infection with MR S. Typhimurium DT 104 has been associated with the consumption of chicken, beef, pork sausages, and meat paste and to a lesser extent with occupational contact with infected animals. This particular clone has subsequently caused outbreaks of infection in food animals and humans in numerous European countries and as far afield as South Africa, the United Arab Emirates, and the Philippines (Threlfall, 2000). In 1996 infections with MR DT 104 were recognized in cattle and humans in North America, both in Canada and in the United States. Of particular concern has been the resistance of the organism to a wide range of therapeutic antimicrobials. Furthermore, in some countries there have been reports of an apparent predilection of the organism to cause serious disease, although this is not the case in the UK.
In the United States, closely related strains of MR S. Newport with plasmid-encoded resistance to ceftriaxone have been associated with numerous infections in both cattle and humans. This has caused it to become the third most common serotype causing salmonellosis in man in the United States from 2000 to 2002. The organism commonly shows resistance to ACSSuT with additional resistance to third-generation cephalosporins mediated by the CMY-2 beta-lactamase gene (see ‘Developed countries’ under ‘Other salmonella serovars’).
Because of the association of many nontyphoidal salmonella serovars with food-production animals, many outbreaks have been linked to foods of animal origin or to foods contaminated with animal waste. This, coupled with the massive importation of foods between countries, including both developed and developing countries, has ensured that products contaminated with salmonella organisms have been widely distributed. An enormous diversity of vehicles of infection have been identified, ranging from salad vegetables to spices to coconut, as well as traditional animal-associated products such as poultry and poultry products, milk, cheese, and undercooked beef and pork. The EU-funded Enter-net surveillance network has identified over 50 such food-borne salmonella outbreaks since its inception in 1992 (Fisher and Threlfall, 2005; Cook et al., 2006), and, in many cases, has been instrumental in the development of intervention measures that have either contained existing outbreaks or have resulted in the removal of contaminated products from the food chain.
A further significant source of salmonellosis in developed countries is pet reptiles. Many reptiles carry salmonella organisms as part of their normal bacterial flora and, although the types of salmonella in reptiles are not those normally associated with large outbreaks, such as those caused by S. Enteritidis, the reptile-associated types can cause very serious illness, particularly in vulnerable patient groups such as young children or the elderly. There have been many cases of reptile-associated salmonellosis documented worldwide. In the United States in the 1970s, a large outbreak involving several thousand people was attributed to turtles; as a result, turtle hatching was banned in the United States in 1975.
